
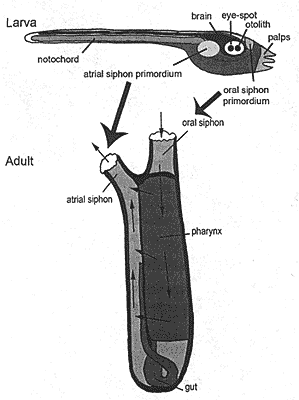
 Сравнение личиночной и взрослой стадий urochordate Ciona intestinalis. Взаимоотношения между зачатками сифонов у личинок и их действительным положением у взрослых. Стрелки показывают направление тока воды во время фильтрации пищи взрослыми.
Сравнение личиночной и взрослой стадий urochordate Ciona intestinalis. Взаимоотношения между зачатками сифонов у личинок и их действительным положением у взрослых. Стрелки показывают направление тока воды во время фильтрации пищи взрослыми.|
|
||||||
|
JOURNAL OF EXPERIMENTAL ZOOLOGY (MOL DEV EVOL) 304B
|
347-399
|
(2005)
|
||||
|
|
||||||
|
|
||||||
|
|
||||||
|
Evolutionary Origins of Vertebrate Placodes: Insights From Developmental Studies and From Comparisons with Other Deuterostomes
|
||||||
|
|
||||||
|
GERHARD SCHLOSSER*
Brain Res.Institute, University of Bremen, FB2, 28334 Bremen, Germany
|
||||||
|
|
||||||
|
ABSTRACT Ectodermal placodes comprise the adenohypophyseal, olfactory, lens, profundal, trigeminal, otic, lateral line, and epibranchial placodes. The first part of this review presents a brief overview of placode development. Placodes give rise to a variety of cell types and contribute to many sensory organs and ganglia of the vertebrate head. While different placodes differ with respect to location and derivative cell types, all appear to originate from a common panplacodal primordium, induced at the anterior neural plate border by a combination of mesodermal and neural signals and defined by the expression of Six1, Six4, and Eya genes. Evidence from mouse and zebrafish mutants suggests that these genes promote generic placodal properties such as cell proliferation, cell shape changes, and specification of neurons. The common developmental origin of placodes suggests that all placodes may have evolved in several steps from a common precursor. The second part of this review summarizes our current knowledge of placode evolution. Although placodes (like neural crest cells) have been proposed to be evolutionary novelties of vertebrates, recent studies in ascidians and amphioxus have proposed that some placodes originated earlier in the chordate lineage. However, while the origin of several cellular and molecular components of placodes (e.g., regionalized expression domains of transcription factors and some neuronal or neurosecretory cell types) clearly predates the origin of vertebrates, there is presently little evidence that these components are integrated into placodes in protochordates. A scenario is presented according to which all placodes evolved from an adenohypophyseal-olfactory protoplacode, which may have originated in the vertebrate ancestor from the anlage of a rostral neurosecretory organ (surviving as Hatschek's pit in present-day amphioxus). J. Exp. Zool. (Mol. Dev. Evol.) 304B:347-399, 2005. © 2005 Wiley-Liss, Inc.
|
||||||
|
|
||||||
|
In a now classic study, Northcutt and Gans ('83; see also Gans and Northcutt, '83; Northcutt, '96, 2005) pointed out that many of the evolutionary novelties of the vertebrate head, including specialized sense organs, the peripheral nervous system, and the craniofacial skeleton, arise from two embryonic tissues, the neural crest and ectodermal placodes. Northcutt and Gans proposed that these embryonic tissues originated in the context of a shift of life histories from filter feeding to more active predation at the outset of the evolution of vertebrates. With recent progress in developmental genetics, the genetic networks underlying neural crest and placode development have begun to be unravelled (reviewed in Baker and Bronner-Fraser, 2001; Mayor and Aybar, 2001; Knecht and Bronner-Fraser, 2002; Gammill and Bronner-Fraser, 2003; Bhattacharyya and Bronner-Fraser, 2004; Streit, 2004). Thus, comparing the expression of genes implicated in
|
neural crest and placodal development between vertebrates and protochordates [i.e., the non-vertebrate chordates: urochordates (tunicates) and cephalochordates (amphioxus)] allows construction of scenarios of the developmental changes leading to the origin of crest and placodes (reviewed in Baker and Bronner-Fraser, '97; Holland and Holland, '99, 2001; Holland et al., 2004; Meulemans and Bronner-Fraser, 2004, 2005; Stone and Hall, 2004), even though, unfortunately, functional data concerning the role of such
|
|||||
|
Grant sponsor: German Science Foundation; Grant number: SCHL 450/5-1, SCHL 450/5-3, SCHL 450/5-4
* Correspondence to: Gerhard Schlosser, Brain Research Institute, AG Roth, University of Bremen, FB 2, P.O. Box 330440, 28334 Bremen, Germany. E-mail: gschloss@uni-bremen.de
Received 14 February 2004; Accepted 26 April 2005
Published online 7 July 2005 in Wiley InterScience (www. interscience.wiley.com). DOI: 10.1002/jez.b.21055.
|
||||||
|
|
||||||
|
|
|||
|
genes in cephalo- and urochordates are usually lacking.
In this paper, which focusses exclusively on placodes, I provide an overview of the phylogenetic distribution of cellular and molecular components of placodes, and discuss implications for our understanding of placode evolution. In the first part, I briefly introduce the different placodes and the diverse array of their derivatives, and then discuss evidence that all placodes develop from a common panplacodal primordium. This primor-dium is defined by the expression of Six and Eya genes. I suggest that these genes promote generic properties and processes in all placodes. I then briefly review how this panplacodal primordium is induced in early vertebrate development. The common developmental origin of different placodes suggests that they also evolved from a common precursor. In order to elucidate the evolutionary origin of placodes, in the second part, I review the phylogenetic distribution of gene expression patterns and cell types involved in vertebrate placode development throughout the deuterostomes, and then evaluate the evidence for the existence of placode homologues in uro- and cephalochordates. Finally, developmental and phylogenetic data are taken together to construct a tentative scenario of placode evolution.
PLACODES CONTRIBUTE TO MANY
EVOLUTIONARY INNOVATIONS OF THE
VERTEBRATE HEAD
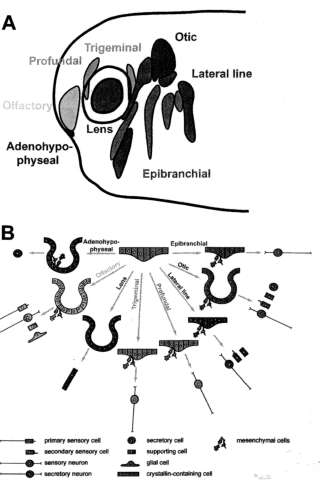
The ectodermal placodes (Fig. 1A) comprise the adenohypophyseal, olfactory, lens, trigeminal, and profundal placodes, a series of epibranchial placodes, hypobranchial placodes (only in amphibians; Schlosser et al., '99; Schlosser and Northcutt, 2000; Schlosser, 2003), an otic placode, and a series of lateral line placodes (lost in amniotes; reviewed in Webb and Noden, '93; Northcutt, '96; Baker and Bronner-Fraser, 2001; Schlosser, 2002a; Streit, 2004). Placodes were originally described as thickenings of the non-neural ectoderm (van Wijhe, 1883; von Kupffer, 1891, 1895). However, while placodes often appear as distinct thickenings, this is not always true; conversely, not every cranial ectodermal thickening can be considered a placode (see discussion in Schlosser, 2002a). More generally, placodes are specialized areas of the cranial non-neural ectoderm (i.e., ectoderm outside of the neural plate), where cells undergo pronounced changes in cell shape (resulting in thickening, invagination, and/
|
 |
||
|
Fig. 1. Ectodermal placodes in vertebrates. (A) Schematic overview of the different types of ectodermal placodes as distributed in a tailbud stage Xenopus embryo (modified from Schlosser and Northcutt, 2000). (B) Schematic summary of morphogenesis and cellular derivatives of various ectodermal placodes. Adenohypophyseal, olfactory, lens, and otic placodes invaginate. In all placodes except the lens placode, cells leave the placodal epithelium as mesenchymal cells to form secretory cells, glial cells, or sensory neurons. In lateral line placodes, another subset of cells forms the lateral line primordia composed of migratory cells, which migrate along the basement membrane.
or cell delamination) and which give rise to various non-epidermal cell types. Hairs, feathers, and teeth (Pispa and Thesleff, 2003) as well as the amphibian hatching gland and cement gland (Drysdale and Elinson, '92; Sive and Bradley, '96) also arise from specialized ectodermal regions showing similar specializations, but these are not counted among the placodes in the strict sense employed here because they do not appear to share a common developmental origin or bias (see below) with the placodes
|
|||
|
|
|||
|
|
||||
|
EVOLUTIONARY ORIGIN OF VERTEBRATE PLACODES
|
349
|
|||
|
|
||||
|
sensu stricto. For example, in amphibians, which have a bilayered ectoderm, cement gland and hatching gland arise from the superficial ectodermal layer (Drysdale and Elinson, '92; Sive and Bradley, '96), which does not express the generic placodal genes Sixl and Eyal, while placodes arise from the deep ectodermal layer, which do express these genes (David et al., 2001; Schlosser and Ahrens, 2004).
Overview of different placodes
As Fig. 1 illustrates, placodes are heterogeneous in regard to both their locations in the head and the different organs they develop into. The adenohypophyseal placode gives rise to the adeno-hypophysis (Couly and Le Douarin, '85; Eagleson et al., '86, '95; Kawamura and Kikuyama, '92; ElAmraoui and Dubois, '93; Kouki et al., 2001; Herzog et al., 2003; Sasaki et al., 2003; Uchida et al., 2003) with six types of endocrine secretory cells: gonadotropes (LH and FSH), thyrotropes (TSH), corticotropes (ACTH), melanotropes (MSH), lactotropes (prolactin—PRL), and somato-tropes (somatotropin) (reviewed in Dubois et al., '97; Kawamura and Kikuyama, '98; Kioussi et al., '99; Sheng and Westphal, '99; Scully and Rosen-feld, 2002; Asa and Ezzat, 2004; Ooi et al., 2004).
The olfactory placode gives rise to the olfactory and vomeronasal epithelia with secretory cells (supporting cells and mucus-producing cells) and the sensory cells bearing odorant and pheromone receptors (e.g., Klein and Graziadei, '83; Couly and Le Douarin, '85; Hansen and Zeiske, '93; reviewed in Brunjes and Frazier, '86; Farbman, '94; Buck, 2004). The sensory cells are primary sensory cells (i.e., they posses axons), which coalesce into the olfactory and vomeronasal nerves. The olfactory placode also gives rise to glia and is the only placode known to do so (Couly and Le Douarin, '85; Chuah and Au, '91; Norgren et al., '92). Neurosecretory cells containing GnRH and other neuropeptides (e.g., FMRFamide, neuropeptide Y) also originate from the olfactory placode and subsequently migrate into various locations in the brain (Schwanzel-Fukuda and Pfaff, '89; Wray et al, '89; Nishimatsu et al, '92; reviewed in Tarozzo et al, '95; Daikoku, '99; Wray, 2002), where they form the terminal nerve-septo-preoptic GnRH system. The septo-preoptic subset of these neurons controls the release of gonadotropins (LH, FSH) from the adenohypo-physis (reviewed in Muske, '93; Parhar, 2002; Somoza et al, 2002; Wray, 2002), whereas another
|
subset forms a distinct ganglionated cranial nerve, the terminal nerve (reviewed in Muske and Moore, '88; Demski, '93; Von Bartheld, 2004), which has neuromodulatory effects on olfactory receptor neurons and/or other neuroendocrine cells (Abe and Oka, 2000; Eisthen et al, 2000; Park and Eisthen, 2003). Recent lineage tracing experiments in zebrafish suggest that the gonadotropin-releasing and terminal nerve-related GnRH cells originate respectively at the boundaries between olfactory and adenohypophyseal placodes and between olfactory placodes and neural crest (Whitlock et al, 2003). However, it is not clear which of these tissues give rise to GnRH cells since the olfactory placode is closely apposed both to the adenohypophyseal placode and to-neural crest.
The lens placode gives rise to the crystallin-containing cells of the lens (reviewed in McAvoy, '80; Piatigorsky, '81; Cvekl and Piatigorsky, '96; McAvoy et al, '99; Ogino and Yasuda, 2000; Chow and Lang, 2001).
The profundal and trigeminal placodes together with neural crest cells contribute sensory neurons to the ganglia of the profundal and trigeminal nerves, respectively (Knouff, '27, '35; Hamburger, '61; Ayer-LeLievre and Le Douarin, '82; D'Amico-Martel and Noden, '83; Artinger et al, '98; Schlosser and Northcutt, 2000), which relay somatosensory information (pain, temperature, and touch) from the rostralmost face and the oral cavity. Here I follow the terminology of Northcutt C93a), who considers profundal and trigeminal nerves as separate cranial nerves, because they have separate cranial ganglia in many gnathos-tomes (e.g., Northcutt and Bemis, '93; Piotrowski and Northcutt, '96), although they fuse during amphibian development (Northcutt and Brandle, '95; Schlosser and Roth, '97). In amniotes, there is usually only a single ganglion, referred to as the trigeminal or Gasserian ganglion, but its ophthalmic and maxillomandibular divisions each receive contributions from a distinct placode (Hamburger, '61; Ayer-LeLievre and Le Douarin, '82; D'Amico-Martel and Noden, '83; Begbie et al, 2002). The ophthalmic and maxillomandibular placodes express different combinations of transcription factors and are probably homologous to the anamniotic profundal and trigeminal placodes, respectively (see discussions in Schlosser and Northcutt, 2000; Schlosser and Ahrens, 2004).
The otic placode gives rise to the entire inner ear and to the sensory neurons of the vestibulocochlear ganglion supplying it (reviewed in
|
|||
|
|
||||
|
|
||||
|
350
|
G. SCHLOSSER
|
|||
|
|
||||
|
Fritzsch et al, '98, 2002; Torres and Giraldez, '98; Fekete and Wu, 2002; Whitfield et al., 2002; Riley and Phillips, 2003; Barald and Kelley, 2004). The inner ear comprises a wide array of specialized epithelial cells including the endolymph-produ-cing secretory cells, supporting cells, and the axonless secondary sensory cells (hair cells)— mechanoreceptive cells distributed among multiple sensory areas dedicated to the perception of sound, gravity, or angular acceleration (reviewed in Miiller and Littlewood-Evans, 2001; Frolenkov et al., 2004; Coffin et al., 2005).
The lateral line placodes, which develop both rostrally and caudally to the otic placode, generate the receptor organs of the lateral line system as well as the sensory neurons of the lateral line ganglia innervating them (reviewed in Winkl-bauer, '89; Northcutt, '92, '97; Smith, '96; Schlos-ser, 2002a; Ghysen and Dambly Chaudiere, 2004). Receptor organs of the lateral line are either mechanoreceptive (neuromasts) or electrorecep-tive (ampullary organs or tuberous organs) and contain secondary sensory cells lacking axons (e.g., hair cells in neuromasts) and secretory supporting cells (reviewed in Flock, '67; Russell, '76; Blaxter, '87; Gibbs, 2004; Coffin et al, 2005). Outgroup comparison suggests that gnathostomes originally had six lateral line placodes (Northcutt, '92, '93a, b, '97), but these placodes have been partially or completely lost several times during gnatho-stome evolution, for example in amniotes (reviewed in Schlosser, 2002b).
The epibranchial placodes, which develop dorso-caudal to the pharyngeal pouches, give rise to viscerosensory neurons in the distal ganglia of the facial (geniculate ganglion), glossopharyngeal (petrosal ganglion), and vagal nerves (nodose ganglia), which supply taste buds and other visceral sensory receptors. In contrast, the neural crest-derived proximal ganglia of these nerves harbor somatosensory neurons (Yntema, '37, '43, '44; Narayanan and Narayanan, '80; Ayer-LeLievre and Le Douarin, '82; D'Amico-Martel and Noden, '83; Couly and Le Douarin, '90; Kious et al, 2002). The hypobranchial placodes in amphibians (Schlosser et al, '99; Schlosser and Northcutt, 2000; Schlosser, 2003) develop ventro-caudal to the pharyngeal pouches and give rise to hypobranchial ganglia. The function of these ganglia is unknown, but they may also be viscerosensory, because the development of hypobranchial placodes suggests that they are merely ventrally displaced epibranchial placodes (Schlosser, 2003).
|
Similarities between different placodes
Although different placodes give rise to different cell types and structures, there are some remarkable similarities between the various placodes. First, placodes are hot spots of cell proliferation, compared with the surrounding epidermal ectoderm (Saka and Smith, 2001). Second, development of all placodes depends crucially on cell shape changes, which underly (1) establishment of focal placodal thickenings, (2) invagination of the adenohypophyseal, olfactory, lens, and otic placodes, (3) elongation or migration of lateral line primordia between surface ectoderm and basement membrane, and (4) epithelial-mesenchymal transitions accompanying the generation of progenitor cells for sensory neurons (profundal, trigeminal, otic, lateral line, epibranchial, and hypobranchial placodes) or for neuroendocrine and glial cells (olfactory placode), which then migrate away and congregate in the adjacent mesenchyme (see Webb and Noden, '93; Baker and Bronner-Fraser, 2001; and other reviews cited above). And third, all placodes, except for the adenohypophyseal and lens, are neurogenic, i.e., they give rise to neurons (e.g., Fode et al, '98; Ma et al, '98; Schlosser and Northcutt, 2000; Andermann et al, 2002; Begbie et al, 2002; Zou et al, 2004). However, in Xenopus, expression of the neuronal determination gene Ngnr-1 is initiated in the ectoderm giving rise to the adenohypophyseal and lens placodes, but subsequently turns off there (Schlosser and Ahrens, 2004). This suggests that the development of these two non-neurogenic placodes may require active inhibition of neurogenesis in ectoderm already biased for a neuronal fate.
While these similarities may be superficial, they more likely reflect the fact that some common aspects of placodal development are goverened by similar gene regulatory networks. Although we still know little about the mechanisms of placode development, recent findings, reviewed in the following sections, indicate that different placodes originate from a common ectodermal region defined by the expression of transcription factors, which promote generic placodal properties shared by all placodes.
DEVELOPMENT OF ALL PLACODES FROM A PANPLACODAL PRIMORDIUM
Despite over 100 years of study, it is still controversial whether all placodes arise from a common primordium (e.g., Piatt, 1894; Knouff,
|
|||
|
|
||||
|
|
|||
|
'35; Nieuwkoop, '63, '85; Jacobson, '66; Torres and Giraldez, '98; Baker and Bronner-Fraser, 2001; Schlosser, 2002a; Streit, 2004) or not (Northcutt and Brandle, '95; Graham and Begbie, 2000; Begbie and Graham, 2001). We and others have argued that two prerequisites must be met for a panplacodal primordium to exist (Schlosser, 2002a; Schlosser and Ahrens, 2004; Streit, 2004): first, all placodes must arise from a single contiguous precursor region (pre-placodal region), and second, this region must be biased towards the development of generic placodal properties shared by all placodes. Bias denotes more than mere competence to respond to inducers; it is rather an autonomous developmental tendency (e.g., resulting from the expression of transcription factors that promote generic placodal properties).
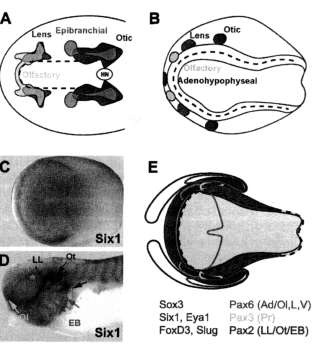
Evidence that the first prerequisite is met comes from fate maps of early embryos of zebrafish (Kozlowski et al., '97), amphibians (e.g., Vogt, '29; Rohlich, '31; Carpenter, '37; Fautrez, '42; Jacob-son, '59; Eagleson and Harris, '90; Eagleson et al., '95), and chicks (Couly and Le Douarin, '85, '87, '90; Streit, 2002; Bhattacharyya et al., 2004) (Fig. 2A and B). These fate maps suggest that all placodes originate from a crescent-shaped pre-placodal region in the outer neural folds and immediately adjacent ectoderm.
It is more difficult to establish whether the second prerequisite is met. Traditionally, the presence of ectodermal thickening was considered as evidence for placodal bias. Indeed, in some vertebrates, the pre-placodal region is characterized by a common placodal thickening around the neural plate and neural crest, but this is not true in other taxa (reviewed in Schlosser, 2002a). I have argued elsewhere (Schlosser, 2002a) that since there is no clear correlation between the distribution of placodes or pre-placodal ectoderm and ectodermal thickenings, the presence or absence of a common ectodermal thickening is an unreliable indicator of placodal bias.
The expression in the pre-placodal region of many transcription factors with demonstrable roles in various aspects of placode development is more elucidating (reviewed in Baker and Bronner-Fraser, 2001; Riley and Phillips, 2003; Streit, 2004; Schlosser and Ahrens, 2004). However, expression of most of them either does not cover the entire pre-placodal region or includes ectodermal domains beyond its boundaries. Only Sixl/2, Six4/5, and Eya genes are expressed in crescent-shaped ectodermal domains (Fig. 2C-E), closely corresponding to the pre-placodal region (Oliver
|
 |
||
|
Fig. 2. All placodes develop from a panplacodal primordium. (A,B) Fate maps of neural plate stage showing the position of some prospective placodes in chick (A) and salamander (Ambystoma) (B) embryos. Hatched lines indicate the border of the neural plate (here denned as the precursor of the entire neural tube, which includes the inner neural folds). All placodes map to a horseshoe-shaped region within and immediately adjacent to the outer neural folds. (A) Extensive overlap between various prospective placodes has been demonstrated in recent studies in the chick embryo (based on data from Streit, 2002; Bhattacharyya et al., 2004). (B) Only a coarse map is available for salamander embryos (based on Carpenter, '37). A recent study (Northcutt, '96) has mapped the otic and lateral line placodes to the lateral neural folds and, thus, to slightly more medial positions than depicted here. (C-E) The expression of Sixl and Eyal defines the panplacodal primordium as shown here for Xenopus (modified from Schlosser and Ahrens, 2004). As illustrated for Sixl, these genes are first expressed in a horseshoe-shaped domain around the neural plate (C) and later continue to be expressed in all placodes at tailbud stages (D). (E) shows position of the Sixl and Eyal expressing panplacodal primordium relative to expression of transcription factors specific for the neural plate (medial Sox3 domain), neural crest (FoxD3, Slug), and various placodes {Pax6, Pax3, Pax2, lateral, and rostralmost medial Sox3 domain). Note that color code for expression domains in (E) does not correspond to color code for placodes in (A-D). The hatched line again indicates the neural plate border. Ad: adenohypophyseal placode; EB: epibranchial placodes; L: lens placode; LL: lateral line placodes; 01: olfactory placode; Ot: otic placode; Pr: profundal placode; V: trigeminal placode. The asterisk in (D) indicates the position of the fused profundal and trigeminal ganglia (the respective placodes are already disappearing at this stage).
et al., '95a; Ohto et al., '98; Seo et al, '98; Esteve and Bovolenta, '99; Sahly et al., '99; Kobayashi et al., 2000; Pandur and Moody, 2000; Ghanbari
|
|||
|
|
|||
|
|
||||
|
352
|
G. SCHLOSSER
|
|||
|
|
||||
|
et al., 2001; Ozaki et al., 2001; McLarren et al., 2003; Bessarab et al, 2004; Schlosser and Ahrens, 2004). These genes subsequently continue to be transcribed in all placodes (although some are downregulated in the lens placode), and appear to promote similar developmental processes in them, including cell proliferation, morphogenetic movements, and neuronal differentiation. This is discussed in more detail in the following section. Thus, the expression of these genes in the pre-placodal region may indeed provide this region with a bias for the development of generic placodal properties, thereby defining a panpla-codal primordium.
Existence of a generic placodal bias in this region is strengthened by experimental overex-pression of transcription factors normally expressed during the development of subsets of placodes. For example, over expression of Pax6, Six3 (each expressed in the adenohypophyseal, olfactory, and lens placode), or Sox3 (expressed in all except profundal and trigeminal placodes) led to the formation of ectopic lenses only in the vicinity of other placode-derived structures in the cranial ectoderm (Oliver et al., '96; Altmann et al., '97; Chow et al., '99; Koster et al., 2000; Lagutin et al., 2001).
Several transcription factors, which function in the development of only some placodes including Pax6 (adenohypophyseal, olfactory, lens, trigeminal), Pax3 (profundal placode), and Pax2 and Pax8 (posterior placodal area giving rise to otic, lateral line, and epibranchial placodes), are already expressed at neural plate stages in specific (but possibly overlapping) subregions of the pre-placodal ectoderm (Piischel et al., '92a, b; Li et al., '94; Stark et al., '97; Pfeffer et al., '98; Baker et al., '99; Streit, 2002; Schlosser and Ahrens, 2004). This suggests that the pre-placodal ectoderm is regionalized before it subdivides into individual placodes (Fig. 2E; Schlosser and Ahrens, 2004), even though cell movements between different subregions still take place (Whitlock and Wester-field, 2000; Streit, 2002; Bhattacharyya et al., 2004).
However, with a few exceptions (e.g., Pax3 expression in the profundal placode), all transcription factors with regionalized expression in the pre-placodal ectoderm are subsequently expressed in multiple placodes (see Schlosser and Ahrens, 2004). For example, Pax6 is expressed in several anterior placodes (adenohypophyseal, olfactory, lens, trigeminal), Irol (trigeminal, profundal, posterior placodal area) and Tbx2 (lens, trigem-
|
inal, profundal, posterior placodal area) are expressed in different subsets of posterior placodes, while Sox2 and Sox3 are expressed in both some anterior (adenohypophyseal, olfactory, lens) and posterior (posterior placodal area) placodes, but not in between.
Individual placodes, thus, appear to be defined by the expression of a unique combination of transcription factors before they begin to express placode-specific markers (as discussed in Schlosser and Ahrens, 2004). Although the precise role of these transcription factors for placode specification remains to be elucidated, these patterns lend some support to the idea that combinatorial codes of genes specify placodes (e.g., Torres and Giraldez, '98) and suggest that placodes arise by a complex multistep induction process involving the partially overlapping activities of several inducers (see below).
A GENERIC ROLE OF SIX1, SIX4, AND EYA GENES FOR PLACODE DEVELOPMENT
The Six- Eya- Pax- Dach network
Six genes are homologues of the Drosophila sine oculis gene, which plays an important role in the development of the compound eye. They encode homeodomain transcription factors that bind directly to DNA and can act as transcriptional repressors or activators depending on the cofac-tors they interact with (reviewed in Relaix and Buckingham, '99; Kawakami et al., 2000; Wawer-sik and Maas, 2000; Hanson, 2001; Epstein and Neel, 2003; Kardon et al., 2004). There are three subfamilies of Six genes—the Sixl/2, Six3/6, and Six4/5 subfamilies—and only one representative of each subfamily was probably present in the last common ancestor of bilaterians (Kawakami et al., 2000; Bebenek et al., 2004) and of chordates (Wada et al., 2003). Only the Sixl/2 and Six4/5 genes exhibit panplacodal expression in vertebrates (Oliver et al, '95a; Ohto et al., '98; Seo et al., '98; Esteve and Bovolenta, '99; Kobayashi et al., 2000; Pandur and Moody, 2000; Ghanbari et al., 2001; Ozaki et al, 2001, 2004; Li et al, 2003; McLarren et al., 2003; Zheng et al., 2003; Laclef et al., 2003a; Bessarab et al., 2004; Schlosser and Ahrens, 2004; Zou et al., 2004), whereas Six3/6 genes are expressed only in an anterior subset of placodes (Oliver et al., '95b; Loosli et al., '98; Jean et al., '99; Lopez-Rios et al., '99; Zuber et al., '99; Bernier et al, 2000; Zhou et al., 2000).
Eya genes Eire homologues of the Drosophila eyes absent gene, another essential regulator of
|
|||
|
|
||||
|
|
||||
|
EVOLUTIONARY ORIGIN OF VERTEBRATE PLACODES
|
353
|
|||
|
|
||||
|
compound eye development (reviewed in Relaix and Buckingham, '99; Wawersik and Maas, 2000; Hanson, 2001; Kardon et al., 2004). Most invertebrates only have a single member of the Eya gene family, but there are four members in vertebrates (Duncan et al., 97; Xu et al., '97; Zimmerman et al., '97; Borsani et al., '99; Mazet et al, 2005). Except for Eya3, all Eya genes are widely expressed in cranial placodes. Depending on the species, either a single Eya gene or a combination of several is expressed panplacodally (Abdelhak et al., '97; Duncan et al., '97; Xu et al, '97, '99, 2002; Kalatzis et al, '98; Mishima and Tomarev, '98; Sahly et al, '99; David et al, 2001; McLarren et al, 2003; Schlosser and Ahrens, 2004; Zou et al, 2004).
In Drosophila, sine oculis and eyes absent form a regulatory network with the nuclear protein dachshund and the Pax6-homologue eyeless. This regulatory network is essential for eye development and its component genes synergize in eliciting ectopic eye development if misexpressed in other imaginal discs (reviewed in Gehring and Ikeo, '99; Relaix and Buckingham, '99; Wawersik and Maas, 2000; Kumar and Moses, 2001a). Genes from the Pax and Dachshund (Dach) families are also coexpressed with Six and Eya family genes in many vertebrate structures, for example somitic muscles, the kidney, retina and several placodes including the ear. Moreover, at least some regulatory interactions between these genes appear to be preserved in the various vertebrate expression domains, suggesting that similar regulatory networks operate in vertebrates, although many details remain to be filled in (reviewed in Relaix and Buckingham, '99; Kawakami et al, 2000; Wawersik and Maas, 2000; Hanson, 2001; Kardon et al, 2004).
Direct physical interactions between the Six transcription factor and the cofactor Eya as well as between the two cofactors Eya and Dach are at the core of the network (Chen et al, '97; Pignoni et al, '97; Heanue et al, '99; Ohto et al, '99; Ikeda et al, 2002; Li et al, 2003; Silver et al, 2003). Recently, it has been shown that in the absence of Eya, Six acts as a transcriptional repressor, while binding of Eya converts it into a transcriptional activator, a switch that is (at least in some cases) mediated by Eya's ability to convert Dach from a compressor into a coactivator of Six (Li et al, 2003; reviewed in Epstein and Neel, 2003). Moreover, Six is also able to interact with other cofactors such as the corepressor Groucho, suggesting that its mode of action may be determined by the
|
balance of available activating or repressive cofactors (Silver et al, 2003; Brugmann et al, 2004).
Sixl, Six4, and Eyal in placodal development
The panplacodal expression of Sixl 12 y Six4/5, and Eya genes suggests that these genes may play important roles in the development of different placodes; the phenotypes of various mutants in mouse, human, and zebrafish support this hypothesis. Although placode derivatives appear normal in Six4 mutants (Ozaki et al, 2001), perhaps due to rescue by other Six genes, Sixl and Eyal mutants have similar defects in most structures in which these genes are normally expressed, including most if not all placodes (Table 1). In these mutants, placode-derived structures are generally reduced or absent. The inner ear, for example, arrests at the otic vesicle stage and does not develop properly organized semicircular canals or a cochlear duct. All sensory areas are strongly reduced in size or missing (Whitfield et al, '96; Johnson et al, '99; Xu et al, '99; Li et al, 2003; Zheng et al, 2003; Laclef et al, 2003a; Ozaki et al, 2004; Zou et al, 2004; Kozlowski et al, 2005). Only a small vestibulocochlear ganglion forms, but later degenerates in the mouse mutant (Zou et al, 2004; Kozlowski et al, 2005).
Sensory areas developing from other placodes are similarly affected. The size and number of lateral line neuromasts are reduced in zebrafish dogeared (Eyal) mutants (Whitfield et al, '96; Kozlowski et al, 2005), while the olfactory epithelium is reduced or absent in mouse Sixl mutants (Li et al, 2003; Laclef et al, 2003a; Ozaki et al, 2004). Moreover, the formation of sensory ganglia from all neurogenic placodes is reduced or blocked: trigeminal and profundal ganglia are reduced in size and epibranchial placode-derived ganglia are either reduced or completely absent in mouse mutants of Eyal or Sixl (Xu et al, '99; Zheng et al, 2003; Zou et al, 2004), although they, as well as lateral line ganglia, are normal in zebrafish Eyal mutants (Kozlowski et al, 2005). Moreover, knocking down Eyal experimentally in Xenopus with morpholino antisense oligonucleotides reduces expression of neurogenic marker genes in all neurogenic placodes and placode-derived ganglia (Schlosser, unpublished observations). Similar morpholino knockdowns of Sixl have also been shown to reduce the early embryonic expression of widely expressed placodal
|
|||
|
|
||||
|
|
|||
|
TABLE 1. Deficits ofplacodal and pharyngeal derivatives in Sixl and Eyal mutants and morphants Placodes Sixl Eyal Sixl/Eyal
Panplacodal markers +1 +2
Adenohypophyseal - — +3
Olfactory +3-5 +2
Lens — +6
Trigeminal +7 +2'7
Profundal +7 +2'7
Otic +3-5,7-9 +2,7,10-16 + 8
Lateral line Not determined +2,10,12
Epibranchial +7,8 +2'7'11
Pharyngeal derivatives (e.g. +4,5,9 +13,44,16,17 thymus)
1Brugmann et al. (2004) (Xenopus Sixl morpholino knockdown).
2Schlosser (unpublished observations) (Xenopus Eyal morpholino knockdown).
3Li et al. (2003) (mouse Sixl and Sixl/Eyal mutants).
4Laclef et al. (2003a) (mouse Sixl mutant).
5Ozaki et al. (2004) (mouse Sixl mutant).
6Azuma et al. (2000) (human Eyal mutants).
7Zou et al. (2004) (mouse Sixl and Eyal mutants).
8Zheng et al. (2003) (mouse Sixl and Sixl/Eyal mutants).
9Ruf et al. (2004) (human Sixl mutants).
10Whitfield et al. ('96) (zebrafish dogeared mutant of Eyal).
nXu et al. ('99) (mouse Eyal mutant).
12Kozlowski et al. (2005) (zebrafish dogeared mutant of Eyal).
13Namba et al. (2001) (human Eyal mutants).
14Abdelhak et al. ('97) (human Eyal mutants).
lDJohnson et al. ('99) (mouse Eyal mutants).
16Chang et al. (2004) (human Eyal mutants).
17Xu et al. (2002) (mouse Eyal mutant).
|
|||
|
|
|||
|
markers such as Eyal and Sox11, but later deficits were not analyzed (Brugmann et al., 2004). Moreover, lens defects (cataracts) were also reported in humans, but not mice, mutant for Eyal (Azuma et al., 2000). Finally, the adenohypophysis is strongly reduced in mouse double mutants of Eyal and Sixl (Zheng et al., 2003), although it is nearly normal in single mutants.
Given this broad spectrum of placodal defects in Six1 and Eyal mutants, can we recognize any common themes? There are in fact at least three developmental processes for which these genes appear to be instrumental.
The first is size regulation: Sixl and Eyal mutants show reduced levels of proliferation, while apoptosis is enhanced (Xu et al., '99, 2002; Li et al., 2003; Zheng et al, 2003; Zou et al, 2004; Kozlowski et al., 2005). Moreover, Sixl and Eya genes appear to directly stimulate cell cycle control genes such as CyclinAl and c-Myc, suggesting that both genes are essential for proliferation (Ford et al., '98; Li et al., 2003; Coletta et al., 2004).
A second common theme is the promotion of cell shape changes and morphogenetic movements. In
|
mutants of both Sixl and Eya1, morphogenetic movements associated with the formation of the membraneous labyrinth as well as the delamina-tion of precursors for placodally derived ganglia are abnormal. Defects in proliferation and morphogenetic processes probably also account for deficiencies in thymus and kidney development seen in Sixl and Eya1 knockout mice (Xu et al., '99, 2002, 2003; Li et al., 2003; Laclef et al., 2003a; Ozaki et al., 2004). In accordance with this idea, Sixl has recently been identified as a key metastatic regulator enhancing the invasiveness of cancer cells (Yu et al., 2004).
The third common theme is the promotion of particular pathways of cytodifferentiation, such as myogenesis in the mesoderm (Heanue et al., '99; Laclef et al., 2003b) or the formation of sensory cells and neurons in the ectoderm. Both Sixl and Eyal mutants have reduced numbers of placodally derived sensory cells and ganglionic neurons (see above) and either fail to express neuronal determination and differentiation genes (including Neurogenins and NeuroD) or do not maintain their expression in neurogenic placodes (Zou et al., 2004). In contrast, overexpression of Eyal
|
||
|
|
|||
|
|
||||
|
EVOLUTIONARY ORIGIN OF VERTEBRATE PLACODES
|
355
|
|||
|
|
||||
|
together with Sixl promotes ectopic neurogenesis (Schlosser, unpublished observation).
Thus, Sixl and Eyal tend to promote those properties (e.g., increased proliferation, a capacity for cell shape changes, and neuronal differentiation) that distinguish placodes from epidermal ectoderm. This indicates that Sixl and Eyal function in the regulation of generic placodal properties shared by all placodes, whereas transcription factors with more restricted expression domains (see below) may be responsible for the regulation of traits specific for groups of placodes or individual placodes, such as the formation of hair cells from the otic placode.
MECHANISMS OF PLACODE INDUCTION
As outlined above, individual placodes initially express transcription factors with a generic role in placode development such as Sixl and Eyal together with a distinct combination of other transcription factors before they express additional placode-specific genes later in development (Schlosser and Ahrens, 2004). Specification of each placode appears to require coexpression of many of these transcription factors, each of which is expressed in a different spatiotemporal pattern and, thus, presumably is induced by different signals. This suggests that placode induction is a complex process involving several steps, viz., the induction of generic placodal transcription factors (generic placode induction) as well as various more specific inductive events, some of which may be shared among subsets of placodes.
The presence of molecularly distinct subregions of the panplacodal primordium at neural plate stages in Xenopus together with experimental evidence for specification of olfactory and otic placodes during neural plate stages in anurans (Bando, '30; Choi, '31; Zwilling, '40, '41; Ginsburg, '95; Gallagher et al., '96) suggests that some of these specific inductive events may occur in parallel rather than in sequence with generic placode induction. An unknown signal from the neural plate and/or neural tube, for example, is required for the locally restricted induction of Pax3 in the prospective profundal placode (Stark et al., '97), which is already evident at neural plate stages in Xenopus.
Placode induction is a multistep process
The complex sequence of steps underlying the induction of an individual placode is best studied for the lens and otic placodes (reviewed in
|
Jacobson, '66; Grainger, '96; Ogino and Yasuda, 2000; Baker and Bronner-Fraser, 2001; Chow and Lang, 2001; Noramly and Grainger, 2002; Schlosser, 2002a; Riley and Phillips, 2003; Barald and Kelley, 2004; Bhattacharyya and Bronner-Fraser, 2004; Lang, 2004; Streit, 2004). In both, signals from the early endomesoderm and from the anterior neural plate are required and neural plate-derived signals (including signals from the optic cup in the case of lens induction) are necessary for multiple steps of placodal development. Several signaling molecules important for later steps (during neural plate-neural fold stages) in these inductive cascades have been identified, including bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs) emanating from the optic cup for lens induction (Furuta and Hogan, '98; Wawersik et al., '99; Faber et al., 2001; Le and Musil, 2001; reviewed in Chow and Lang, 2001; Lang, 2004) and various FGFs emanating from the hindbrain for otic induction (Represa et al., '91; Lombardo and Slack, '98; Ladher et al., 2000; Vendrell et al., 2000; Phillips et al., 2001; Leger and Brand, 2002; Maroon et al., 2002; Alvarez et al., 2003; Liu et al., 2003; Wright and Mansour, 2003; reviewed in Noramly and Grainger, 2002; Riley and Phillips, 2003; Barald and Kelley, 2004). FGFs—possibly from the endomesoderm—were also shown to play a role during earlier (gastrula-neural plate) stages of otic induction (reviewed in Noramly and Grainger, 2002; Riley and Phillips, 2003), but the early signals involved in lens induction remain to be identified. Unfortunately, most studies of placode induction have focussed on a single placode without systematically exploring whether and to what extent inductive events are shared between different placodes. For example, many of the transcription factors, implicated specifically in otic placode development and used as otic-specific markers in induction assays, such as Pax2, Pax8, and Foxll, are initially (neural plate/fold stages) expressed in a broad domain termed the posterior placodal area (Schlosser and Ahrens, 2004), which probably gives rise to lateral line and epibranchial placodes in addition to the otic placode. Differential expression of genes between these three types of placodes is only observed at later (tailbud) stages (Schlosser and Ahrens, 2004), suggesting that at least some of the signals implicated in early otic induction may in fact be more general inducers for the posterior placodal area. The latter may only later be subdivided into different types of placodes by the locally restricted action of specific inducers
|
|||
|
|
||||
|
|
|||
|
356 G. SCHL
such as persistent FGF signaling from the hind-brain for the otic placode (reviewed in Noramly and Grainger, 2002; Riley and Phillips, 2003), and BMP 7 from the pharyngeal pouches for the epibranchial placodes (Begbie et al., '99).
Generic placode induction
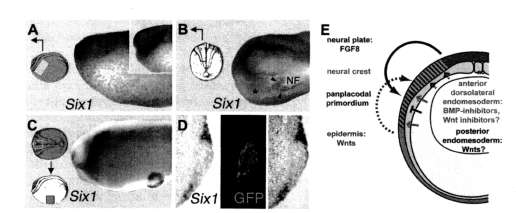
While much remains to be learned about those inductive events, which are shared among some but not all placodes, several recent studies in Xenopus (Brugmann et al., 2004; Glavic et al., 2004; Ahrens and Schlosser, submitted) and chick embryos (A. Litsiou, S. Hanson, and A. Streit, personal communication) have begun to elucidate the tissues and signals involved in generic placode induction, that is in the induction of the panpla-codal primordium itself (Fig. 3).
Both the dorsolateral endomesoderm underlying the pre-placodal ectoderm and the anterior neural plate are required for generic placode induction but their relative importance differs between species. While the neural plate was able to induce some panplacodal markers including Sixl in competent non-neural ectoderm of both Xenopus
|
OSSER
and chick, the endomesoderm was unable to ectopically induce Sixl in Xenopus, but induced an even larger spectrum of panplacodal markers in chick embryos (Ahrens and Schlosser, submitted; A. Litsiou, S. Hanson, and A. Streit, personal communication).
Initially, it was suggested that intermediary BMP concentrations are required for generic placode induction and that an ectodermal BMP gradient positions the panplacodal primordium (Brugmann et al., 2004; Glavic et al., 2004). However, subsequent studies argue against this gradient model in showing that BMP inhibition is necessary for generic placode induction without supporting a requirement for intermediate BMP levels (Ahrens and Schlosser, submitted; A. Litsiou, S. Hanson, and A. Streit, personal communication). In addition, FGF signals (including FGF8 from the anterior neural plate in Xenopus) have been shown to be required for generic placode induction (Ahrens and Schlosser, submitted; A. Litsiou, S. Hanson, and A. Streit, personal communication). Finally, there is evidence that generic placode induction is inhibited in the trunk region by Wnt signals and requires Wnt inhibitors
|
||
|
|
|||
 |
|||
|
|
|||
|
Fig. 3.. Induction of the panplacodal primordium. (A-D) Inductive tissues as revealed by extirpation and transplantation experiments in Xenopus (Ahrens and Schlosser, submitted). Unilateral extirpation of either cranial dorsolateral endomesoderm (A: lateral view; control side shown in inset) or anterior neural plate (B: dorsal view) leads to reduction (asterisks) of placodal Sixl expression, indicating that signals from both tissues are necessary for induction of Sixl (arrowheads in (B) indicate residual Sixl expression at remnants of neural folds on experimental side). (C,D) Transplantation of the anterior neural plate from a donor embryo labeled with green fluorescent protein (GFP) to the belly of a host embryo leads to ectopic induction of Sixl in the host ectoderm (ring around graft in C; section through graft shown in D), indicating that signals from the neural plate are sufficient for induction of Sixl (this is not the case for dorsolateral endomesoderm; not shown). (E) Model for the induction of the panplacodal primordium illustrated in a schematic crosssection through a neural plate stage Xenopus embryo. During gastrulation two ectodermal territories have been established, which differ in competence. Dorsal ectoderm (blue) has a default neural fate but is competent to respond to neural crest inducers (such as Wnts; green and maroon broken arrows) from the paraxial and intermediary mesoderm and the adjacent epidermis with neural crest formation (pink). Ventral ectoderm (green) has a default epidermal fate but is competent to respond to panplacodal inducers from dorsolateral endomesoderm and anterior neural plate. FGF8 from the anterior neural plate (blue arrow) is sufficient to induce the panplacodal primordium when BMPs and Wnts are inhibited. In the cranial region, BMP inhibitors and Wnt inhibitors (orange arrows), probably emanating from the endomesoderm, allow the induction of the panplacodal primordium (red), whereas in the trunk Wnts (maroon bars) repress placode formation (after Ahrens and Schlosser, submitted). Mesoderm: dark gray; endoderm: light gray.
|
|||
|
|
|||